Individual cells are combined to form a battery. These batteries serves as a source of energy to vehicles and other equipment. Let us discuss about the difference between battery and fuel cell in detail.
What is a Battery Cell?
Batteries are made up of one or more cells, each of which produces an electron flow in a circuit through chemical reactions. An anode, a cathode, and an electrolyte are the three basic components of all batteries.
A chemical reaction occurs between the anode and the electrolyte. When the anode and cathode of a battery are connected to a circuit. This reaction sends electrons across the circuit and back to the cathode. This produces the required current flow. When the material in the cathode or anode is completely exhausted, then there is no power available.

Related article
What is a Fuel Cell?
A fuel cell is an electrochemical cell that uses a pair of redox processes to transform the chemical energy of a fuel and an oxidizing agent into electricity. Fuel cells differ from most batteries in that they require a constant supply of fuel and oxygen. As long as fuel and oxygen are available, fuel cells can create power.
Transportation, material handling, and permanent, portable, and emergency backup power are just a few of the applications for fuel cells. Hydrogen can be used in fuel cells to generate electricity through a chemical reaction rather than burning, leaving only water and heat as waste.
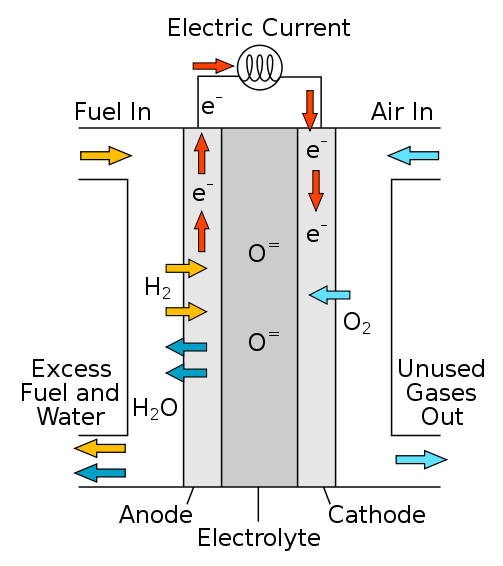
How does a fuel cell work?
Difference between Batteries and Fuel Cells
The below table summarize the difference between fuel cell and battery:
| Battery cell | Fuel cell |
|---|---|
| They store energy in the form of chemical energy | They cannot store energy. Fuel cell converts chemical energy to electrical energy. |
| Reactants are inside the cell itself. | Reactants for chemical reaction are supplied continuously. |
| Chemical reaction products remain inside the cell itself | Chemical reaction products are removed from the cell |
| Rechargeable | Not rechargeable |
| Less efficiency | High efficiency |
| It consists of limited amount of fuel and oxidant and these reactants diminish with time | Needs a continuous supply of fuel and oxygen from an external source |
| Supply energy for a limited period of time | Supply energy for a long period of time |
| Less expensive | They are expensive |
| Example : lithium ion batteries | Example : hydrogen-oxygen fuel cell |



